
Afghanistan 2013 © Vivian Lee/MSF
Antimicrobial resistance
Resistance to antimicrobial medicines, such as antibiotics, has become a global health crisis, complicating the treatment of bacterial infections and endangering lives around the world.
MSF statement and position paper on antimicrobial resistance for UN High-Level Meeting
May 15, 2024 — Doctors Without Borders/Médecins Sans Frontières (MSF) delivered a statement at the United Nations and released an accompanying position paper that lays out recommendations to help address the global health threat of antimicrobial resistance in advance of the UN High-Level Meeting this fall.
Read the statement
Putting antimicrobial resistance (AMR) in context
The ability of all types of microbes (bacteria, viruses, parasites, and others) to survive medicines used against them is called antimicrobial resistance. In the case of bacterial pathogens, for which antibiotics are the most important drugs used in treatment, we speak of antibiotic resistance.
Antimicrobial resistance (AMR) is increasing at alarming rates in countries with failing health systems and poor sanitation, and especially in regions at war. Without urgent action, simple cuts and many diseases could once again become deadly since today’s medicines will no longer work against them. At Doctors Without Borders/Médecins Sans Frontières (MSF) we are finding more and more AMR infections—in patients ranging from victims of war wounds or burns to severely malnourished children and even newborns—and are expanding our activities to prevent and treat them.
of antibiotics used globally
are prescribed incorrectly
newborns die each year
from drug-resistant infections
new class of antibiotics
was developed in the last 30 years
Facts about antimicrobial resistance
Bacteria can become resistant to an antibiotic when they are exposed to it repeatedly, or to incomplete or sub-optimal doses. This can lead to the growth of mutant bacteria which the drug can no longer kill. Resistance occurs in a wide range of disease-causing bacteria and can also be transmitted from one type of bacteria to another.
AMR infections are especially common in settings where off-the-shelf or counterfeit antibiotics are widely available, or where antibiotics are often overused or misused. Without access to proper laboratory diagnostics, health care providers often do not know whether a patient’s symptoms are caused by a bacterial infection, and if so, which type of bacteria is involved. This can lead to unnecessary or incorrect prescriptions for antibiotics. Resistant bacteria can also spread in hospitals with poor sanitation or inadequate infection control, infecting especially vulnerable patients who are already sick or have unhealed wounds.
AMR infections often go unrecognized in their early stages, since they usually cause the same clinical symptoms as antibiotic-sensitive infections. Typically they are identified only after the patient’s symptoms persist despite treatment with appropriate first-line antibiotics.
Prevention of infections is crucial in the fight against AMR. Fewer infections means less antibiotic use, and therefore less chance that bacteria develop resistance or that already-resistant infections spread.
Infection prevention involves many different approaches, such as ensuring widescale population coverage with vaccines against infectious diseases, providing safe water and sanitation in communities, and establishing effective infection control measures in hospitals and health clinics.
When patients seek treatment for illnesses, doctors and health workers must avoid overusing antibiotics, and they need access to microbiology laboratories that can accurately diagnose infections and point doctors to the correct prescription. Patients should be educated about the importance of taking antibiotics only when they are truly necessary, prescribed by a clinician, and provided by a reputable pharmacy, and of taking the prescribed dosage for the prescribed period of time.
Comprehensive infection prevention is challenging since it requires strong commitment and participation by many different stakeholders. Some key steps, such as strengthening laboratory capacity and hospital infection control practices, require investment and good basic infrastructure, while others call for behavioral changes by patients and medical staff alike. And some demand decisive action from policymakers—for example, to end the over-the-counter and black-market antibiotic sale common in many countries.
Antimicrobial resistance is often suspected when a patient’s infection fails to improve after a course of what would usually be an appropriate drug. Confirmation of resistance requires laboratory testing of samples from the patient’s blood, bone, tissue, or cerebrospinal fluid; results of these ‘antibiograms’ also provide information on which alternative antibiotics should cure the infection. But antibiograms are usually not done in low-resource settings, since microbiology laboratories and trained clinical staff may be scarce or non-existent.
Without lab support, clinicians may attempt a diagnosis based on available knowledge about the types of bacterial infections and drug resistance patterns prevalent in the region. But even this information is often lacking. In these cases, physicians often prescribe antibiotics that work against a very wide range of bacteria—leading to overuse of, and fueling resistance to, these precious broad-spectrum drugs.
Patients with an AMR infection, especially one resistant to multiple drugs, have fewer antibiotic treatment options, and these options are generally more expensive and used intravenously rather than as oral drugs.
Antibiotics can be categorized as narrow or broad spectrum. Those with a narrow spectrum target only specific bacterial families, while broad-spectrum antibiotics kill many different types of bacteria. To avoid resistance, narrow-spectrum drugs are the best choice when the type of bacteria causing a patient’s infection is known or strongly suspected and is not life-threatening—for example, in cases of chronic infections. But when a patient is very ill and the cause of infection is unclear, doctors often start treatment right away with a broad-spectrum antibiotic and then switch to a narrower one if a bacterial pathogen is identified. For patients with bloodstream or central nervous system infections, immediate antibiotic treatment can mean the difference between life and death.
For many types of highly resistant infections where no current antibiotics are effective, patients may be treated either with old antibiotics that were abandoned due to their potentially dangerous side effects, or with a combination of antibiotics that together might overcome the resistance. When no antibiotics work and the infection is localized to a limb, amputation may be the only hope for saving the patient’s life.
How MSF responds to antimicrobial resistance
The rise in AMR infections worldwide, combined with MSF’s increased engagement in Middle Eastern countries at war, has made antibiotic resistance a slow-motion emergency facing many of our teams.
For example, in our reconstructive surgery program in Amman, Jordan, about half of all war-wounded patients from Iraq, Syria, and Yemen arrive with serious infections, up to 60 percent of which are AMR. The same holds true in Yemen, where war has destroyed an already fragile health care system, and with it the capacity for proper sterilization, hygiene, and care for patients with open fractures or other severe injuries highly susceptible to infection. Rampant overuse and misuse of antibiotics also helps drive the development and spread of resistant infections in settings like these.
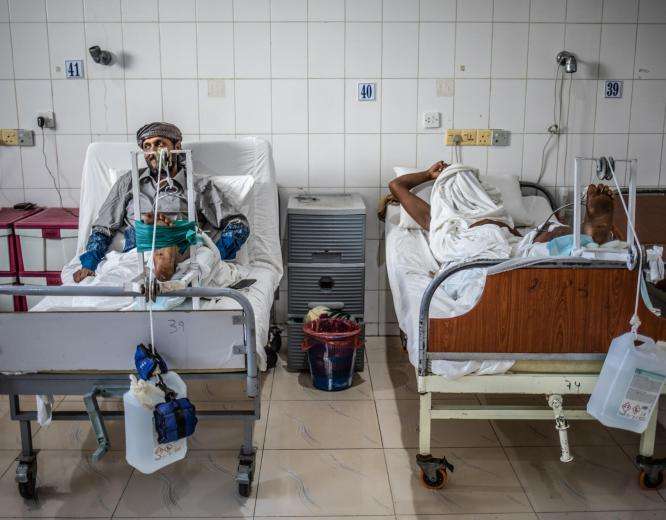
How inequitable access to quality health care drives antimicrobial resistance
Communities where MSF operates are among the most vulnerable to AMR.
More news and stories
Learn about MSF’s journalistic roots and our commitment to bear witness and speak out about the plight of the people we treat.
Research May 23, 2024
Doctors Without Borders releases 2023 activity report on antimicrobial...
Read More
Research Nov 15, 2023
Antimicrobial resistance must be addressed in pandemic preparedness an...
Read MoreLearn about MSF’s journalistic roots and our commitment to bear witness and speak out about the plight of the people we treat.



